It is atmosphere that makes life possible on the planet earth and earth is the only planet in the solar system with such an atmosphere that can sustain life. Atmosphere protects us from the harmful radiation and blasts of heat emanating from the sun.
The blanket of gases (atmosphere) is about 480 kilometers thick. The earth’s atmosphere is divided into five layers: the exosphere, the thermosphere, the mesosphere, the stratosphere and the troposphere.
The second layer as you go up, stratosphere, starts above the troposphere layer and ends about 50 km (31 miles) above ground. This layer provides a very important natural shield that protects us from the harmful ultraviolet radiations from the sun and only let the good rays in. This important natural shield layer of gas is known as the ozone layer.
The ozone layer is about 7 to 25 miles above the Earth’s surface and acts as a “sunscreen” for the planet earth.
What is Ozone?
Ozone (O3) is a colorless gas with its molecules consisting of 3 oxygen atoms and is located in the upper layers of the atmosphere, where it protects us from the sun’s harmful ultraviolet (UV) radiation by absorbing it.
Although it represents only a tiny fraction of the atmosphere about 3-5 mm thick, ozone is crucial for sustaining life on earth. Without ozone, the sun’s intense UV radiation would sterilize the earth’s surface. With a weakening of this shield, more intense UV-B and UV-A radiation exposure at the surface would lead to quicker sunburns, skin cancer, blindness, weak immune system and even reduced crop yields in plants.
Chemical Composition of Ozone (O3)
There are natural processes that create and destroy the ozone layer in the stratosphere. These processes regulate a balance of ozone and form the ozone layer. It’s the sunlight which create ozone, primarily. When high-energy ultraviolet rays (UV-C) strike an oxygen molecule (O2), they split the molecule into two single oxygen atoms, known as atomic oxygen. This process is shown in the figure below.
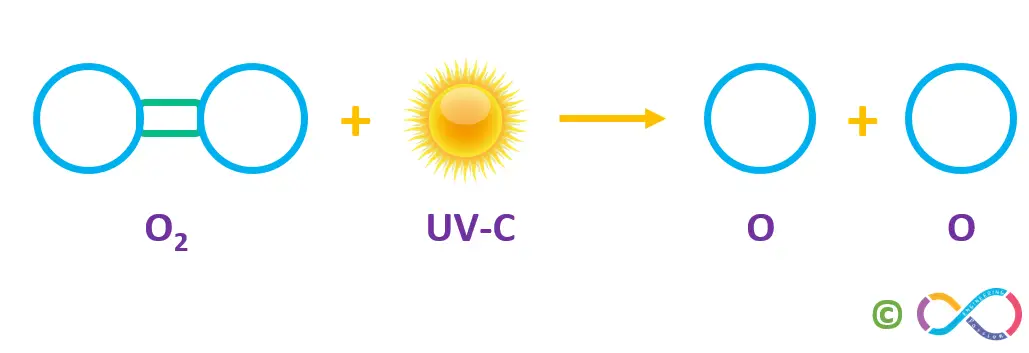
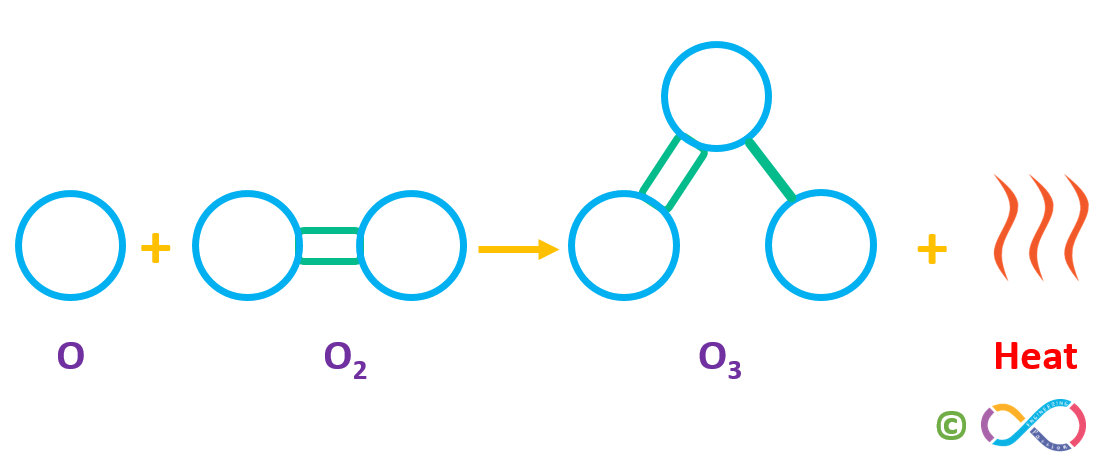
A freed atomic oxygen (O) then combines with another oxygen (O2) molecule to form a molecule of ozone (O3). This “ozone-oxygen cycle” is continuously absorbing high-energy ultraviolet radiation (UV-C) and blocking it completely from reaching the earth’s surface.
This process as shown below, creates heat which warms the upper part of the stratosphere. The air is very dry in the stratosphere and is a thousand time thinner than it is at sea level and this is why balloons and jet aircraft fly here.
Ozone is very reactive and attacks other molecules in the air, often regenerating oxygen in the process (this is why ozone is important to us). Ozone in the stratosphere absorbs much of the sun’s UV-B rays, splitting back into molecular and atomic oxygen as shown below.
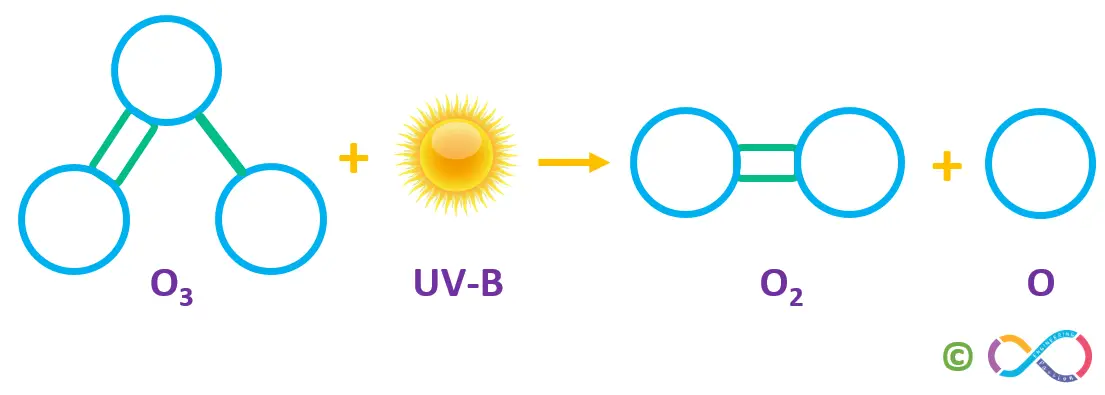
No matter, how the atomic oxygen atoms are produced, they almost always quickly react with oxygen molecules, reforming the ozone.
So, while ozone is continually being created, it is also continually being destroyed. Sometimes an ozone (O3) molecule reacts with an oxygen atom creating two oxygen molecules, thus ending the cycle. If the rate of ozone creation is equal to the rate of destruction, the total amount will remain the same.
Scientists have found that ozone level change periodically with regular natural cycles such as the changing season, winds and long-time scale sun variations. Ozone also responds to some sporadic solar events such as flares. Moreover, volcanic eruptions may inject substances into the stratosphere that can lead to increased destruction of the ozone layer.
Although Ozone (O3) depletion does not cause global warming, but both of these environmental problems have the same cause: human activities that release chemical pollutants into the atmosphere changing it. Human-made chemicals have been poking holes in ozone layer for years as these chemicals can remain in the atmosphere between 50 and 100 years. Ozone is vital for sustaining life on earth, we should protect it.




![Types of Engineers and What they Do [Explained]](https://www.engineeringpassion.com/wp-content/uploads/2022/04/types-of-engineers-and-what-they-do-280x210.jpg)








Leave a Reply